Second-Generation Zaire Ebola Virus Vaccine Development Announced
SK bioscience today announced it is partnering with Hilleman Laboratories Singapore to develop a low-cost, improved manufacturing process, second-generation Ebola-Zaire vaccine.
Currently, Ebola vaccines have been authorized and used in Africa since 2019.
On November 22, 2023, SK bioscience confirmed it will acquire unique expertise and know-how for the use of recombinant Vesicular Stomatitis Virus Vector (rVSV) technology platform in close collaboration with Hilleman Laboratories to potentially jointly develop other vaccines against a variety of viral infectious diseases.
Jaeyong Ahn, CEO of SK bioscience, commented in a press release, "Developing a vaccine to prevent viruses causing diseases with a high fatality rate, such as Ebola-Zaire, is essential for us to protect humanity."
"By cooperating with Hilleman Laboratories for a successful development of the second-generation Zaire Ebolavirus vaccine, we will contribute to overcoming the Ebola Zaire disease burden and expand our cooperation with global companies and institutions."
In 2014, the World Health Organization declared an international public health emergency during the Ebola outbreak and encouraged the development of the vaccine when the virus was spreading rapidly in West Africa.
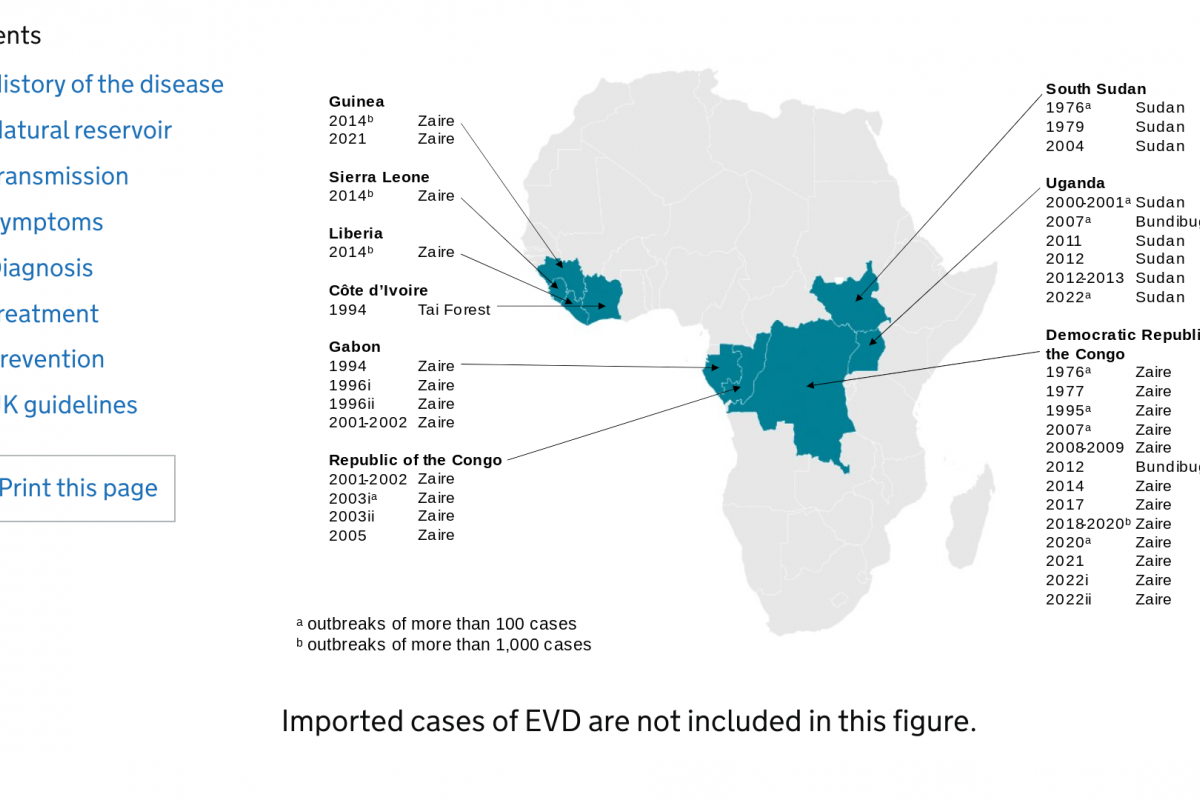
Ebola Virus Disease is a rapidly progressive, severe, and transmissible hemorrhagic illness caused by infection with one of the Ebola Virus (EBOV) species. While there are six identified EBOV species, the Zaire Ebola virus strain has been the leading cause of outbreaks over the last 20 years.
Ever since the Ebola virus was first discovered in 1976, there have been multiple outbreaks resulting in significant loss of lives (50% mortality rate) and economic impact.
Our Trust Standards: Medical Advisory Committee























